The Man Who Smelled Like Rancid Creamed Corn to Usher In a New Scientific Era
Over the past decade, a group of HIV activists has helped pioneer gene therapy.
Updated at 6:27 p.m. ET on December 19, 2018.
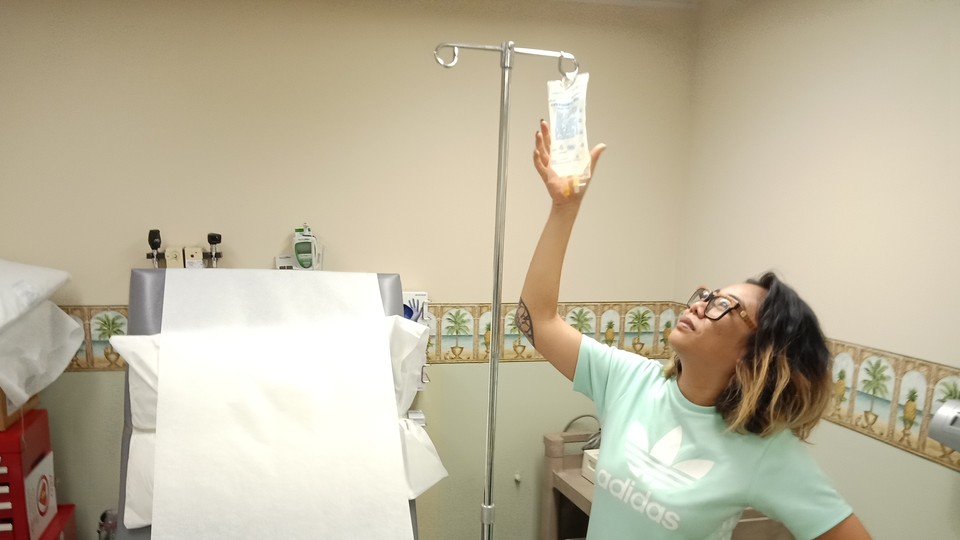
When the nurse slipped the IV needle into his arm, Matt Sharp was calm. Yes, he knew the risks: As one of the first humans ever to receive the experimental treatment, he could end up with mutant cells running amok in his body. But he was too enamored of the experiment’s purpose to worry about that. For two decades, Sharp had been living with HIV. He’d watched the height of the AIDS crisis claim dozens of his friends’ and lovers’ lives. Now, he believed he was taking a step toward a cure.
A few months earlier, researchers had drawn white blood cells from Sharp’s body and manipulated his DNA with tiny molecules, deleting a single gene in each cell. He was about to receive an infusion that would reintroduce the tweaked cells back into his bloodstream. The procedure aimed to change the genetic makeup of these cells to make Sharp’s body resistant to HIV. Gene therapies like this had been tried before for other diseases, but experiments were put on hold when a young man died in 1999. Sharp’s body would allow researchers to test the safety of new molecular tools called “zinc fingers.”
The infusion took place in June 2010 at Quest Clinical Research, a nondescript gray building near The Fillmore in San Francisco. Sharp’s cells arrived frozen in a liquid-nitrogen-filled shipping container that looked like R2-D2 from Star Wars. After thawing them in a hot-water bath, his nurse plugged the bag into his IV line. Cloudy yellow fluid slowly drained into Sharp’s arm. Within 30 minutes, he headed back to work with billions of genetically modified cells reproducing in his arteries and veins.
Over the past decade, HIV patients like Sharp have played a major role in pushing forward the vanguard science of gene editing. The community’s close-knit advocacy networks, paired with the fact that there are clearly identifiable genes that make humans vulnerable to HIV, have made people living with the virus ready candidates for innovative—though sometimes risky—experiments. A gene-editing procedure related to HIV rocked the fields of science and medicine last month, when the Chinese researcher He Jiankui made the explosive claim that he had manipulated the genomes of twin babies who do not carry the virus in an attempt to make them resistant to it, a covert and reckless move that was widely condemned by the scientific establishment. But adult HIV patients have voluntarily participated in scientifically condoned experiments that have paved the way for further gene-editing work on, for instance, cancer and blindness.
Sharp’s journey since he signed up for his pioneering infusion illustrates the potential that DNA editing has in expanding the possibilities of being human—and also the limits of genetic medicine as a miracle cure.
Sharp, who’s now 62, has been campaigning for innovative experimental medicine since the 1980s. As a veteran of the AIDS Coalition to Unleash Power, or ACT UP, he says he was arrested at least eight or nine times—he can’t remember exactly—during protests against slow progress in AIDS treatment and research. He first encountered the founder of Quest Clinical Research, Jay Lalezari, in San Francisco back in the early 1990s, when the young doctor was making a name for himself with daring experiments with potentially toxic drugs. Lalezari’s research offered early hope for surviving HIV, but he initially had an uneasy relationship with activists: ACT UP staged a “die in” at one of his talks at an AIDS conference.
Over time, the activists began to trust Lalezari. Hundreds of people now credit him for saving their lives. In 2008, Lalezari was involved in an earlier gene-therapy study, funded by Johnson & Johnson, that reported unspectacular results. So when a small biotech company, Sangamo Therapeutics, approached him with a gene-editing experiment involving the new zinc-finger technology, he was skeptical that it would be a “cure.” But after careful consideration, he decided to launch an experiment and enroll 65 patients with HIV.
When Sharp learned about the gene-editing trial, he jumped at the chance to participate and agreed to be patient No. 2 in the safety study. To everyone’s relief, Sharp experienced only one side effect after the initial infusion: An intense smell lingered around his body. When he returned to Quest the next day for a checkup, his nurse could smell him coming down the hall. Paperwork from Sangamo had said to expect a garlic odor from a chemical agent that would fade after a few days, but the nurses agreed that Sharp and his fellow gene-editing patients smelled more like rancid creamed corn.
When the earliest results came back, Sharp saw dramatic improvements in his medical charts. For the first time since he was diagnosed with HIV, his T-cell count—a key marker of immune-system function—jumped up to normal, healthy levels. Sharp wanted to be in the audience when the full data were unveiled at a leading HIV conference, so he flew to Boston in February 2011. He didn’t know if his cell counts were an idiosyncratic fluke. When Lalezari presented a slide showing a significant increase in the other patients’ T-cell numbers as well, Sharp says the audience gasped.
Over the next couple of years, follow-up procedures were more uncomfortable for Sharp than the initial infusion. During rectal biopsies, he watched a video screen that showed where “the scope was going into my butt, where the clippers were going, and exactly where they were taking the snips,” he says. He was under local anesthesia and didn’t feel any pain, so he decided to narrate the procedure with an omniscient booming voice-over: “Journey to the Center of the Earth.”
No serious problems were identified among the patients in the study in rectal exams, blood samples, and general checkups. But Sharp’s lack of complications was unusual. Other patients did have notable side effects, including fever, chills, headaches, and muscle pain. According to one study, these symptoms were likely a reaction to billions of cells being reinfused into the body, rather than from the genetic modifications. Still, there are other concerns. The study’s three-year monitoring period might not have been enough to detect long-term health problems, such as cancer, caused by genetic damage from the experiment.
More pointedly, the experiment did not deliver the dream of a complete cure. Some patients experienced no lasting benefits. Sharp found long-term improvements to his immune-system health. He thinks participation in the study was worth it, but he still takes daily pills to keep the virus under control. Other patients claim that they were “cured” and stopped taking standard medicine when their bodies did, in fact, become able to control HIV.
Lalezari insists that it is irresponsible to bandy about the word cure when interpreting results from this initial research. The study was promising, but preliminary; the “primary outcome was safety,” researchers say. Current HIV medications have few risks, can reduce the virus to undetectable levels, and are becoming more affordable. Lalezari points to other HIV experimental treatments—involving antibodies, pharmaceutical drugs, and small molecules—that seem even more promising than gene editing.
The most important next step for Lalezari after his 2011 presentation in Boston was more research. If his studies could, in fact, produce a one-time treatment, as some of his patients claimed, gene-editing truly would be a game changer. But his work abruptly stalled out when it ran into a fickle reality of cutting-edge experimental work: funding troubles. Sangamo Therapeutics, the biotechnology firm that bankrolled the initial study, decided to sideline its HIV research, and instead develop treatments for other diseases with its proprietary gene-editing technology.
This enraged HIV-positive activists. “The company put it back on the shelf because they couldn’t figure out how they were going to make enough money,” says Mark Harrington, the executive director of the Treatment Action Group in New York City. Sharp, dismayed by the setback, signed an open letter to Sangamo, calling for new research initiatives. “They simply refused to follow up the initial experiment with funding for further studies,” he says.
The reasons the funding dried up are disputed. In 2013, Sangamo had become flush with cash when it signed a $320 million deal. But then its shares plummeted as an insider-trading scandal threw the company into disarray. The U.S. Securities and Exchange Commission charged Sangamo’s then–vice president of clinical research, Winson Tang, with allegedly participating in a scheme that netted more than $1.5 million in “illegal profits.”
“When Winson Tang was carted off to jail, Sangamo completely dropped the ball,” contends Lalezari, who says he maintains contact with the biotech firm to leave open the door to future experiments. “If you want to do research with industry, you can never forget that you are dancing with the devil.”
Sandy Macrae, the president and CEO of Sangamo, disputes such an inference, claiming that the decision to move away from HIV came before the Winson Tang insider-trading scandal. But the company acknowledges that it didn’t see HIV as the most valuable investment. Macrae took charge of Sangamo last year, shortly after the company’s stocks bottomed out at around $3 a share, and he has been working to turn things around. The company’s headquarters sits next to a boat-parts store in Richmond, California, a city at the edge of the Bay Area that is also home to a train yard, a Chevron oil refinery, a yacht club, and Rosie the Riveter National Park.
“I had to make a decision about where our portfolio was best applied,” he says, pointing out that existing HIV medicine is already effective. “My company has only so many things we can do. When I looked at HIV, they had done a lot of work trying to get a product. The current version … doesn’t feel like it is there.”
Sharp remains undaunted. After failing to get funds from Sangamo, he began lobbying U.S. government officials and HIV researchers. Thanks in part to his efforts, Rafick-Pierre Sékaly, a professor at the Case Western Reserve University School of Medicine, was inspired to continue gene-editing research on HIV. The National Institutes of Health awarded Sékaly $11 million earlier this year for a new experiment called TRAILBLAZER that pays Sangamo for access to its gene-editing technology.
Sharp “has always been extremely passionate. He has always been very forceful, pushing us to do more,” Sékaly says. Now, a new cohort of HIV patients are joining Sékaly’s experiment. They are helping open up a future where gene editing could be a routine part of medical care.
Exactly what this future might look like remains to be seen. “If we could all be engineered to resist HIV,” Sharp says, “then the stigma associated with the disease might completely disappear.” If the technology becomes more effective, then a wide range of genetic diseases could be fixed. But gene editing carries other risks, besides technical problems. Existing gene therapies are very expensive, up to $475,000 per treatment. Gene editing could produce a new era of inequality in medicine, to say nothing of the fears some people have of “designer babies” or controversies about children engineered to resist HIV from birth.
There are subtler, more surprising possibilities, too. Tim Dean, a queer theorist known for flipping conventional scripts, speculates that gene editing could create a new class distinction among gay men in hook-up culture.* “In some techno-future, I can imagine cruising apps including a category, in addition to HIV status, that would differentiate the edited from the unedited,” he says.
Sharp, for his part, just wants to see research on a cure move forward. He doesn’t care if the cure is DNA editing, an antibody, or a new pharmaceutical drug. “I have already volunteered for 12 clinical trials,” he says, “and I am willing to try anything new if it looks like it might work.”
* This article previously misstated Tim Dean’s HIV status. We regret the error.